ü Ammonia:
Ø Preparation: In laboratory, ammonia is obtained by treating ammonium salts with
caustic soda (NaOH) or slaked lime.
(NH4)2SO4 + 2NaOH → 2NH3 + 2H2O
+ Na2SO4
2NH4Cl + Ca(OH)2 → 2NH3 + 2H2O
+ CaCl2
On a large scale, ammonia is manufactured by Haber’s
process.
N2(g) + 3H2(g)
→ 2NH3(g)
In accordance with Le Chatelier’s
principle, high pressure of about 200 atm, a temperature of about 773 K and the
catalyst such as iron oxide with small amounts of K2O and Al2O3
are employed to increase the rate of this reaction.
Ø Properties:
· Ammonia is a colourless gas with pungent smell.
· It is highly soluble in water because of its ability to form
inter molecular hydrogen bond with water.
· Liquid
ammonia has high melting and boiling points because of inter molecular hydrogen bonding.
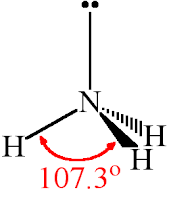
· The ammonia molecule has a trigonal pyramidal geometry.
It has three bond pairs and one lone pair of electrons.
·
Its
aqueous solution is weakly basic due to the formation of OH– ions.
NH3(g) + H2O(l) → NH4+
(aq) + OH– (aq)
·
As a weak
base, it precipitates the hydroxides
of many metals from their salt solutions. For example,
2FeCl3 (aq )+ 3NH4OH
(aq ) →
Fe2O3.xH2O (s) + 3NH4Cl
(aq)
ZnSO4 (aq)+ 2NH4OH
(aq) →
Zn(OH)2 (s) + (NH4)2SO4
(aq)
·
The
presence of a lone pair of
electrons on the nitrogen atom of the ammonia molecule makes it a Lewis base.
It donates the electron pair and forms complex compounds with Cu2+,
Ag+ etc. So, it is
used for the detection of these metal ions.
Cu2+ (aq) + 4 NH3(aq) → [Cu(NH3)4]2+(aq)
(blue) (deep blue)
Ag+ ( aq) + Cl− (aq) → AgCl (s)
(colourless)
(white ppt)
AgCl (s) + 2NH3 (aq ) →
[Ag (NH3)2]Cl (aq)
(white ppt) (colourless)
Ø Uses: Ammonia is used
i) To produce various nitrogenous fertilizers (ammonium nitrate,
urea, ammonium phosphate and ammonium sulphate)
ii)
In the manufacture of nitric acid.
iii)
liquid
ammonia is used as a
refrigerant.
iv) As a laboratory reagent.
v)
In the production of artificial rayon, silk, nylon
etc.
vi) For manufacture of HNO3
by the Ostwald process.

0 Comments