Quantitative Aspects of electrolysis – Faraday’s
laws
ü Faraday’s first law:
The
amount of substance(m) deposited at the electrode during electrolysis is
directly proportional to the quantity of electricity(q) passed through
electrolyte.
m α
Q
OR
m = ZQ
Where
z is a constant called electrochemical equivalent. Z = equivalent weight/96500
But
quantity of electricity is the product of current in ampere (I) and time in
second (t).
i.e. Q = It
Therefore, m= Zit
ü Faraday’s second law:
When
same amount of electricity is passed through different electrolytic solution,
amount of Substance deposited is proportional to the chemical equivalent weights.
For e.g. when same quantity of electricity is passed through solutions
of two substances A and B, thenMass of A deposited = Equivalent mass of A
Mass of B deposited Equivalent mass of B
Products
of electrolysis: The
products of electrolysis depend on the nature of the electrolyte and the type
of electrodes used. If the electrode is inert (e.g. Pt, gold etc.), it does not
participate in the electrode reaction. While if the electrode is reactive, it also
participates in the electrode reaction.
For
e.g. if molten NaCl is electrolysed, Na is deposited at the cathode and
chlorine is liberated at the anode. NaCl → Na+ + Cl-
At
cathode: Na+ + e- → Na
At
anode: Cl- → ½ Cl2 + e-
Batteries:
A
battery is basically a galvanic cell in which the chemical energy of a redox
reaction is converted to electrical energy. They are of mainly 2 types –
primary batteries and secondary batteries.
ü Primary
batteries: Here the reaction occurs only once and after use over a
period of time, they become dead and cannot be reused. E.g. Dry cell, mercury
button cell etc.
(Primary cells cannot
be recharged and reused).
v Dry
Cell
v Mercury
Cell
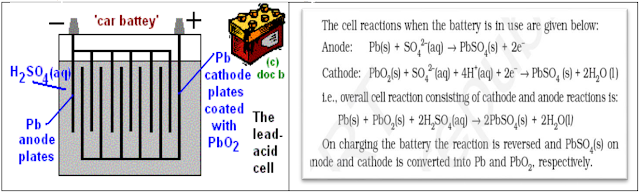
ü Secondary cells: A
secondary cell can be recharged and reused again and again. Here the cell
reaction can be reversed by passing current through it in the opposite
direction. The most important secondary cell is lead storage cell, which is
used in automobiles and invertors.
v Lead – Storage Battery
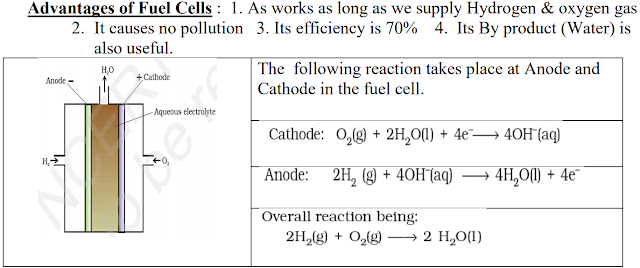
Fuel Cells: These are galvanic cells which
convert the energy of combustion of fuels like hydrogen, methane, methanol,
etc. directly into electrical energy.
One example for fuel cell is Hydrogen – Oxygen fuel cell, which is
used in the Apollo space programme. Here hydrogen and oxygen are bubbled
through porous carbon electrodes into concentrated aqueous sodium hydroxide
solution. To increase the rate of electrode reactions, catalysts like finely
divided platinum or palladium metal are filled into the electrodes.
Corrosion: It is a process of eating away of metals on their
surfaces, it is an unwanted process as it results in loss of mass of metals. In
this process metal surface reacts with atmospheric oxygen to form a layer of
oxide. It is an electrochemical reaction.
Most
familiar example for corrosion is rusting of iron. It occurs in presence of
water and air. It is a redox reaction. At a particular spot of the metal,
oxidation takes place and that spot behaves as anode. Here Fe is oxidized to Fe2+.
2 Fe(s)→2 Fe2+ +4 e–
Electrons
released at anodic spot move through the metal and go to another spot on the
metal and reduce oxygen in presence of H+. This spot behaves as
cathode. The reaction taking place at this spot is:
The
overall reaction is: 2Fe(s)+O2(g)
+ 4H+(aq) → 2Fe2 +(aq)+ 2 H2O (l )
The ferrous ions (Fe2+) are further
oxidised to ferric ions (Fe3+) and finally to hydrated ferric oxide
(Fe2O3. x H2O), which is called rust.
Methods
to prevent corrosion:
ü By
coating the metal surface with paint, varnish etc.
ü By
coating the metal surface with another electropositive metal like zinc,
magnesium etc. The coating of metal with zinc is called galvanisation and the
resulting iron is called galvanized iron.
ü By
coating with anti-rust solution.
ü An
electrochemical method is to provide a sacrificial electrode of another metal
(like Mg, Zn, etc.) which corrodes itself but saves the object (sacrificial
protection)
Related Post:
Electrochemistry - Part 4
Electrochemistry - Part 3
Electrochemistry - Part 2
Electrochemistry - Part 1






0 Comments