Chapter – 5
Surface Chemistry
ü Introduction
ü Adsorption
Ø Adsorbent
Ø Adsorbate
ü Desorption
ü Sorption
ü Mechanism
of Adsorption
ü Types
of Adsorption
Ø Physisorption
(Physical Adsorption)
Ø Chemisorption
(Chemical Adsorption)
ü Adsorption
Isotherm
ü Applications
of Adsorption
ü Catalysis
Ø Homogeneous
and Heterogeneous catalysis
ü Adsorption
Theory of Heterogeneous Catalysis
ü Shape
Selective Catalysis
ü Enzyme
Catalysis
ü Colloids
Ø Classification
of Colloids
ü Preparation
and Purification of Colloids
ü Emulsion
ü Colloids
around us
Surface Chemistry is the branch
of chemistry that deals with the study of the phenomenon occurring at the
surface than bulk.
ü Adsorption: The accumulation of molecular species at the surface rather than in the bulk of a solid or liquid is termed as adsorption.
Ø Adsorbate: The
substance which is adsorbed is called adsorbate.
Ø Adsorbent:
The substance whose surface on which adsorption takes place is called
adsorbent.
The commonly used adsorbents are charcoal, silica gel,
alumina gel, clay, colloids, metals in finely divided state etc.
Adsorption is a
surface phenomenon. Some examples of adsorption are:
1.
Powdered
charcoal adsorbs gases like H2, O2, CO, Cl2,
NH3, SO2 etc.
2.
Silica
gel adsorbs moisture
3.
Animal
charcoal adsorbs colouring material from sugar solutions.
ü Desorption:
The
removal of the adsorbed substance from a surface is called desorption.
ü Sorption: If adsorption and absorption occur simultaneously,
the process is called sorption.
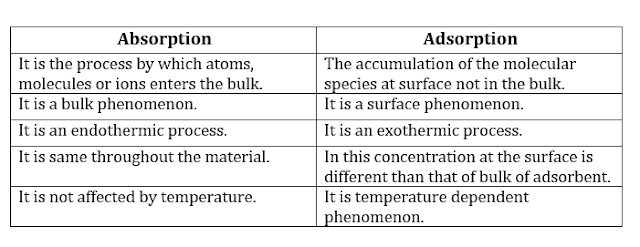
ü Distinction
Between Adsorption and absorption:
ü Mechanism of Adsorption:
The
surface particles of the adsorbent are not in the same environment as the
particles inside the bulk (inner part). Inside the adsorbent, all the forces
are mutually balanced. But at the surface, there is always some unbalanced or
residual forces. These forces of the adsorbent are responsible for adsorption.
ü Gibbs energy change during adsorption:
During
adsorption, there is always decrease in residual force i.e., there is decrease
in surface energy, which appears as heat. Therefore, adsorption is an
exothermic process i.e. ΔH = – ve. Also, movement of the particles are
restricted in this process. Therefore ΔS = – ve According to Gibbs Helmholtz
equation:
ΔG
= ΔH – TΔS, or ΔG = (–ΔH ) – T(–ΔS) for adsorption to
occur.
ΔG must be negative which is possible
only when ΔH >TΔS.
ü Types of Adsorption:
Ø Physical
adsorption or Physisorption: When the particles of adsorbate are held
to the surface of adsorbent by weak van der Waals forces.
Characteristics
of physical adsorption: Lack of specificity, low enthalpy of
adsorption, reversible in nature, no activation energy required, decrease with
increase in temperature, multilayered phenominan.
Ø Chemical
adsorption or Chemisorption: When the molecules of adsorbate are held
to the surface of adsorbent by strong chemical forces.
Characteristics
of chemical adsorption: Highly specific in nature, high
enthalpy of adsorption, Irreversible in nature, initially it increases with
increase in temperature as it needs activation energy, very slow, monolayered phenominan.


0 Comments